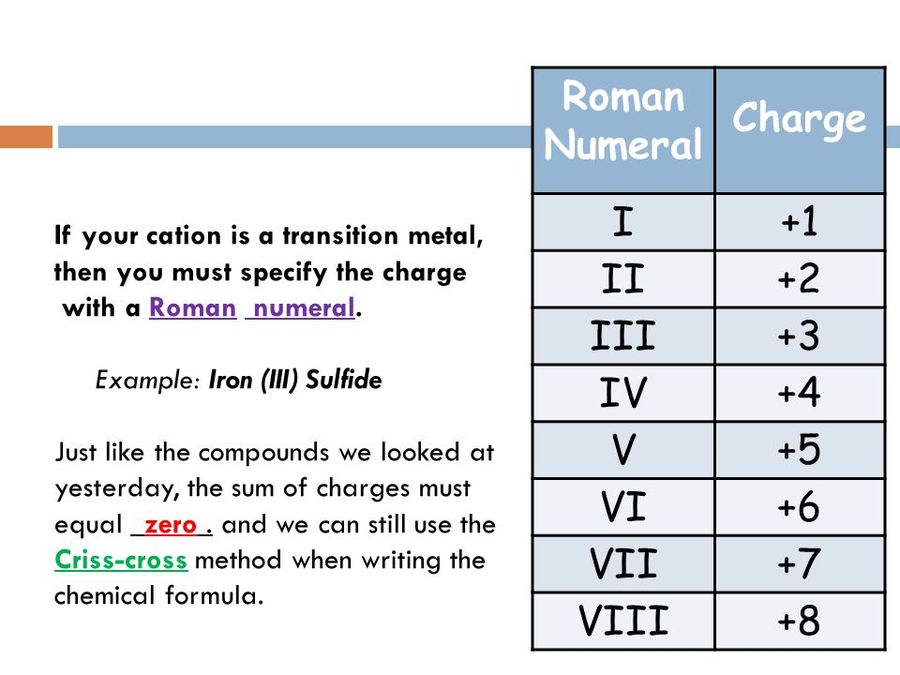
What Does the Roman Numeral Represent in a Chemical Name
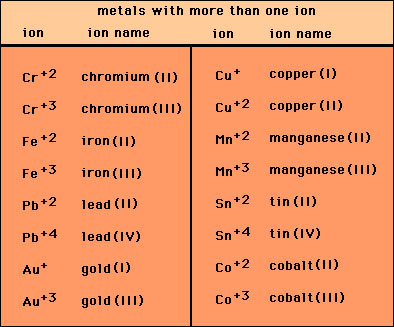
They are used in situations where the multiple oxidation states are available to the metal. For example iron can form two common ions Fe2 and Fe3.

Inorganic Chemistry Groups Of The Periodic Table Chemistry Stack Exchange
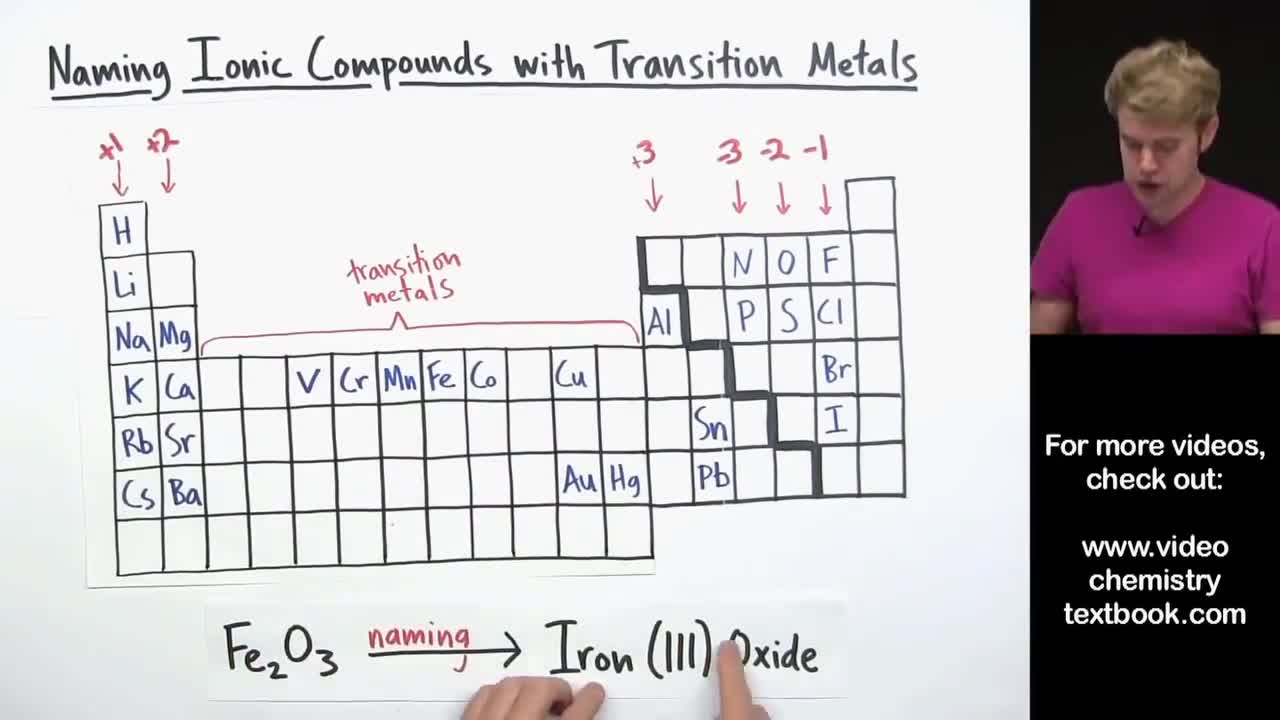
All metals except Al Zn and those in Groups 1 and 2 can have more than one oxidation number.

. Lead is in Group 4 so we. Roman numerals numbers are sometimes used in formula eg. Group IIA elements have two valence electrons and so forth.
The Roman numerals on a periodic table of elements define the chemical group of the elements in that column and identify the number of valence electrons of each element. Group IA elements have one valence electron. When we name their compounds we have to specify which oxidation number is involved.
In chemistry nomenclature writing names systematically Roman numerals are used for a specific group of elements. 1 Answer Ernest Z. So for example if we have something like copper too nitrate the two represents the charge on the copper cat eye on so the correct chemical formula would be See you having a two plus charge would require to nitrates which each have.
Answer 1 of 6. Name of metal oxidation number in parentheses name of anion. Beside above when naming ionic compounds when do you use Roman numerals.
The VN of lead in the example above is 2Roman numerals are commonly used to indicate a different valency than the Group would give eg. These elements are called transition metals. The Roman numeral II represents the number 2.
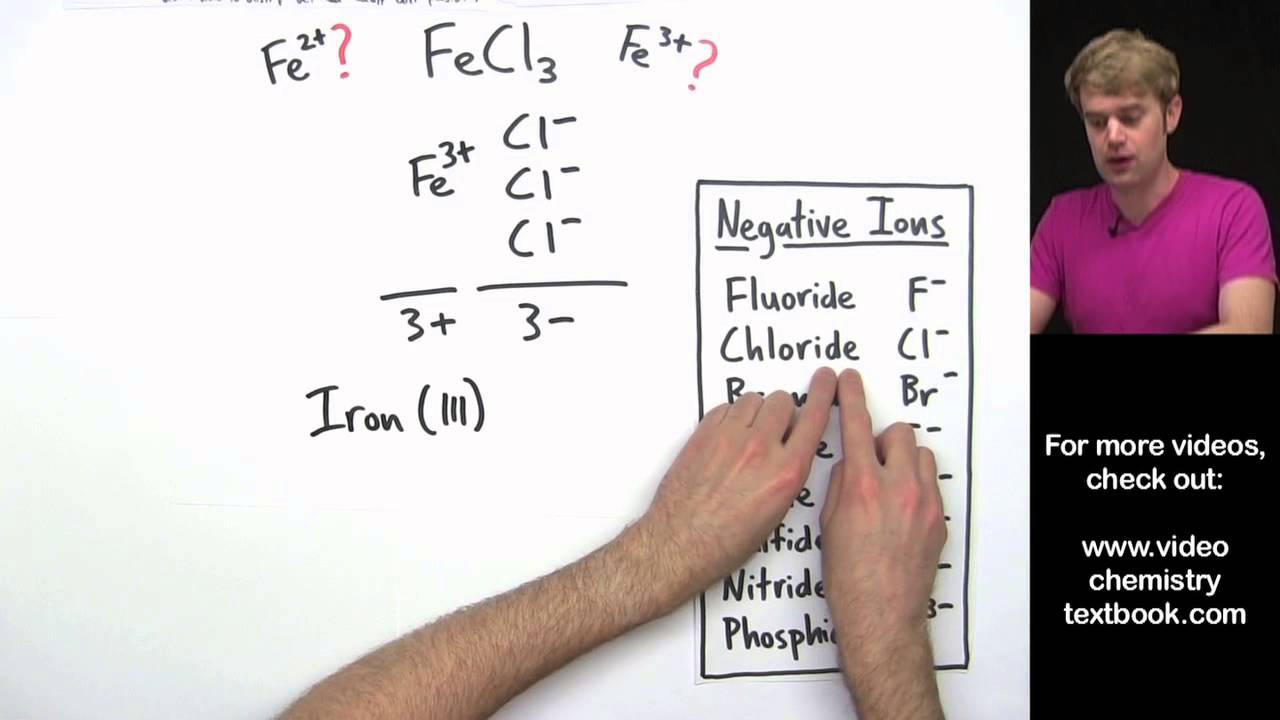
There are rules for oxidation state of elements for example oxygen will almost always have an oxidation state of -2. For example iron can form two common ions Fe2 and Fe3. The charge of the metal.
The example for the case when the Roman numerical in a name is the subscript in the formula. What does the Roman numeral after the metal name represent. Provide two examples of the use of Roman numerals in pharmacology.
It is not necessarily the same as the subscript of the molecular formula. The Stock comes from the name of Alfred Stock a German chemist who first proposed a new way of naming compounds based on the oxidation state of the metal 1919. This means in MnO 2 there are 2 oxygens of oxidation state -2 giving them a combined oxidation state of -4.
People also ask what do Roman numerals in chemistry mean. Higher charge the elements ending with -ic have a higher charge than ones ending in -ous. They refer to the valency of the metal in the compound such as Iron II or Iron III chloride.
And the Roman numerals indicate the charges that these metals carry in a compound. Metal ends in ic does it refer to the ion with the higher or lower charge. They are used to identify narcotic classifications and also on some written prescriptions.
What do the roman numeral in a chemical formula mean. Write the equivalent of 0300 using civilian time. What do the roman numeral in a chemical formula mean.
They refer to the valency of the metal in the compound such asIron II or Iron III chloride. The Roman numeral denotes the charge and the oxidation state of the transition metal ion. Since each element within a group has the same number of valence.
This means to balance the. Roman numerals in a chemical formula indicate the charge on the metal cation before them. Rules for naming Type II binary ionic compounds.
Metal is the charge on the transition metal in the Ionic compound. Why is the military time keeping system preferred in the healthcare setting. The Roman numeral denotes the charge and the oxidation state of the transition metal ion.
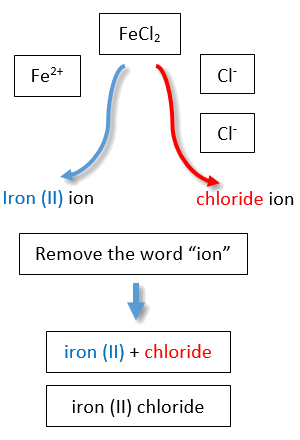
LeadII chlorideWhen they are used they are there to indicate the valency number VN of the element they are next to. The Roman numeral in the names of certain compounds is part of the Stock system of chemical nomenclature. The Roman numeral in the name of the compound does not represent the number of ions present in the compound but represents the charge on the ion thus.
To distinguish the difference Fe2 would be named iron II and Fe3 would be named iron III. Is iron iii nitrate a molecular or. To distinguish the difference Fe2 would be named iron II and Fe3 would be named iron III.
The roman numerals refer to the charge on that metal or element this is referred to as its oxidation state. The Roman numeral in each name represents the charge on the ion and allows us to distinguish between. The Roman numeral in the name of an Ionic compound that contains the transition.
Apr 24 2015 You name ionic compounds with Roman numerals according to the format.

Dublin Schools Lesson Naming Ionic Compounds

Solved Table 4 Chemical Name Chemical Formula Febr2 Febrz Chegg Com

Dublin Schools Lesson Naming Ionic Compounds
Naming Compounds With Transition Elements Quizizz

How To Write Formula With Transition Metals Kealakehe High School

Naming Compounds Using Roman Numerals Youtube
Why Is The Charge Of Transition Metals Written In Roman Numerals Quora

Anyone Knows Why Do They Put That Bracket And A Roman Numeral In The Name Of The Compounds Brainly In
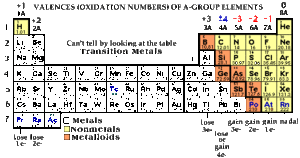
4 Steps To Naming Compounds In Chemistry Nomenclature By Ernest Wolfe Countdown Education Medium
4 Steps To Naming Compounds In Chemistry Nomenclature By Ernest Wolfe Countdown Education Medium
What Does The Roman Numerals Mean In A Chemical Compound Quora

Ionic Compound Formulas Examples How To Write Formulas For Compounds Video Lesson Transcript Study Com

Naming Ionic Compounds With Roman Numerals Youtube
Elemental Ions And Simple Salts
4 7 Naming Ionic Compounds Chemistry Libretexts
Naming Ionic Compounds With Transition Metals Kealakehe High School
Why Is The Charge Of Transition Metals Written In Roman Numerals Quora
Comments
Post a Comment